Scientists have implicated a type of stem cell they believe is responsible for calcified blood vessels common in patients with chronic kidney disease. The research will guide future studies into ways to block minerals from building up inside blood vessels and exacerbating atherosclerosis, the hardening of the arteries.
The study, led by researchers at Washington University School of Medicine in St. Louis, appears Sept. 8 in the journal Cell Stem Cell.
“In the past, this calcification process was viewed as passive — just mineral deposits that stick to the walls of vessels, like minerals sticking to the walls of water pipes,” said senior author Benjamin D. Humphreys, MD, PhD, director of the Division of Nephrology and an associate professor of medicine. “More recently, we’ve learned that calcification is an active process directed by cells. But there has been a lot of controversy over which cells are responsible and where they come from.”
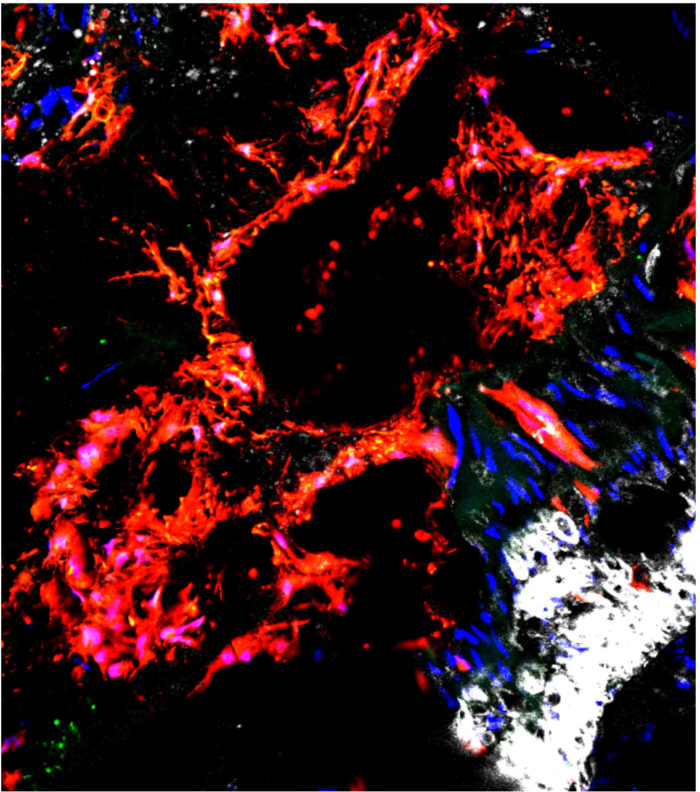
The cells implicated in clogging up blood vessels with mineral deposits live in the outer layer of arteries and are called Gli1 positive stem cells, according to the study. Because they are adult stem cells, Gli1 cells have the potential to become different types of connective tissues, including smooth muscle, fat and bone.
Humphreys and his colleagues showed that in healthy conditions, Gli1 cells play an important role in healing damaged blood vessels by becoming new smooth muscle cells, which give arteries their ability to contract. But with chronic kidney disease, these cells likely receive confusing signals and instead become a type of bone-building cell called an osteoblast, which is responsible for depositing calcium.
“We expect to find osteoblasts in bone, not blood vessels,” Humphreys said. “In the mice with chronic kidney disease, Gli1 cells end up resembling osteoblasts, secreting bone in the vessel wall. During kidney failure, blood pressure is high and toxins build up in the blood, promoting inflammation. These cells may be trying to perform their healing role in responding to injury signals, but the toxic, inflammatory environment somehow misguides them into the wrong cell type.”
The researchers also studied donated tissue from patients who died of kidney failure and who showed calcification in the aorta, the body’s largest artery.
The study’s first author Rafael Kramann, MD, a former postdoctoral fellow in the Humphreys Lab, commented that“We found Gli1 cells in the the calcified aortas of patients in exactly the same place we see these cells in the mice. This is evidence that the mice are an accurate model of the disease in people.”
About 20 million adults in the U.S. have some degree of chronic kidney disease, according to the Centers for Disease Control and Prevention. But most of these patients never develop late-stage kidney failure that requires dialysis or kidney transplantation because they succumb to cardiovascular disease first, Humphreys said. The buildup of plaque in the arteries that is characteristic of cardiovascular disease is worsened in patients with diseased kidneys because of the additional mineral deposits.
Further supporting the argument that Gli1 cells are driving the calcification process, Humphreys and his colleagues showed that removing these cells from adult mice prevented the formation of calcium in their blood vessels.
“Now that we have identified Gli1 cells as responsible for depositing calcium in the arteries, we can begin testing ways to block this process,” Humphreys said. “A drug that works against these cells could be a new therapeutic way to treat vascular calcification, a major killer of patients with kidney disease. But we have to be careful because we believe these cells also play a role in healing injured smooth muscle in blood vessels, which we don’t want to interfere with.”
Humphreys is continuing to focus on the kidney in studying ways to guide Gli1 cells away from bone-building osteoblasts and toward vessel-healing smooth muscle cells. Dr. Kramann, who is now at Aachen University in Germany, plans to investigate the same process with a focus on the heart.
Julia Evangelou Strait
###
This work was supported by the German Research Foundation, grant number KR-4073/3-1; the European Research Council, grant number ERC-StG 677448; a START Grant from the RWTH Aachen University (101/15); the State of North Rhine-Westphalia; the National Institutes of Health (NIH), grant numbers DK088923, DK103740, DK103050, P30 CA91842 and UL1 TR000448; and the American Heart Association, grant number EIA14650059.
Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, Gratwohl S, Madhurima K, Hutcheson JD, Jain S, Aikawa E, Humphreys BD. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell. Sept. 8, 2016.